Abstract
Background: Cytokine release syndrome (CRS) is a common phenomenon that typically occurs post haploidentical hematopoietic stem cell transplant (haplo-HSCT). Patients often develop fevers due to the alloreactive T cells after infusion. CRS has been associated with worse transplant outcomes. Studies have demonstrated improved transplant outcomes with early initiation of immunosuppression, but the etiology of this is unclear. We aim to assess the incidence of haplo-storm and transplant outcomes of our modified post-transplant cyclophosphamide (PTCy) based graft-versus-host disease (GVHD) prophylactic regimen which initiates immunosuppressive therapy earlier than the standard schedule.
Objective: To compare our novel GVHD regimen to our previous standard GVHD regimen and analyze the change in CRS and transplant outcomes.
Study Design: This is a retrospective cohort study of 90 patients who received haplo-HSCT with PTCy GVHD prophylaxis from Intermountain Healthcare's Blood and Marrow Transplant (BMT) Program. In November 2020, the BMT Program changed the haplo-HSCT GVHD prophylaxis regimen. Prior to the change, patients would receive PTCy on days +3 and +4, with MMF and a CNI starting on day +5. Our novel regimen included changing PTCy to be given on days +3 and +5, with an earlier introduction of the CNI and MMF, starting on day -1 and 0 respectively. The vast majority (96%) of patients were treated with peripheral blood stem cells (PBSC).
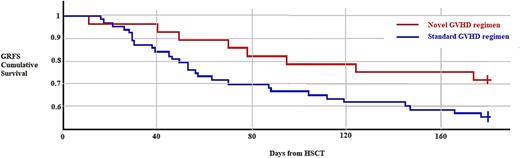
Results: In the 7 days post-transplant, 59/90 patients met criteria for CRS. The incidence of CRS in the novel group was 26% and 83% in the standard group (P <0.0001). There was also significantly higher GRFS when comparing patients with CRS vs without CRS (75% and 51.7%, P = 0.0483). Mean duration of CRS symptoms was significantly shorter in patients receiving the novel GVHD prophylaxis regimen, 1.44 days vs 3.14 days (P = 0.00026). Hospital stay from time of transplant to discharge was significantly lower (22 vs 29.05, P = 0.0241). No adverse outcomes were associated with early initiation of immunosuppression.
Conclusions: This is the first analysis to out knowledge to show a difference in rates and duration of CRS associated with early initiation of immunosuppressive therapy. We found low rates of CRS in patients who were treated almost exclusively with PBSC graft source. Early initiation of immunosuppression during haplo-HSCT did not adversely affect outcomes. CRS was significantly lower in our novel GVHD regimen group with improved GRFS in patients who did not experience CRS and a trend towards improved GRFS in all patients who received the novel GVHD prophylactic regimen. Patients also had earlier time to discharge from date of infusion with earlier immunosuppression. These results suggest that the link between early immunosuppression and improved clinical outcomes may be via reduction in the incidence of CRS. Larger studies are needed to fully validate these outcomes.
Disclosures
Hunter:Kite Pharma: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Notable Labs: Consultancy, Honoraria; Genmab: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal